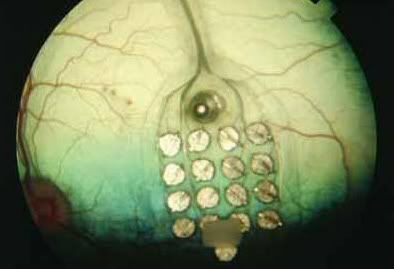
 “An implanted, sight-enhancing device some are calling a ‘bionic eye’ is the first to gain approval for use in the United States,” according to a Midwest Lens post. “According to the U.S. Food and Drug Administration, the new Argus II Retinal Prosthesis System can help patients with a genetic eye disease called retinitis pigmentosa regain some sense of vision. About 100,000 Americans are believed to be affected by the illness, which causes a gradual deterioration of the eyes’ photoreceptor cells.” Read more.
“An implanted, sight-enhancing device some are calling a ‘bionic eye’ is the first to gain approval for use in the United States,” according to a Midwest Lens post. “According to the U.S. Food and Drug Administration, the new Argus II Retinal Prosthesis System can help patients with a genetic eye disease called retinitis pigmentosa regain some sense of vision. About 100,000 Americans are believed to be affected by the illness, which causes a gradual deterioration of the eyes’ photoreceptor cells.” Read more.
Tuesday, April 2, 2013
FDA Approves ‘Bionic Eye’ for Patients with Retinitis Pigmentosa
Subscribe to:
Post Comments (Atom)

No comments:
Post a Comment